Ivermectin vs FDA
Attempts to portray Ivermectin as a product that is exclusively developed for the deworming of animals and that it is being widely adapted in livestock dosage for COVID is completely untrue
To begin with, there is a safe and effective dosage of Ivermectin for human consumption and it has been adapted toward treatment and as a prophylactic to prevent the Chinese Coronavirus.
If you were to believe the FDA, you would think that a great majority of those who are troubled by the reporting of results and efficacy of the Big-Pharma produced compounds (from Pfizer, Moderna, and J&J) are seeking unsafe alternatives.
The FDA appears to be implying that to combat the Chinese Coronavirus, there are many across America who are rushing to purchase and ingest the large livestock dosage of Ivermectin because it is unregulated and easily available.
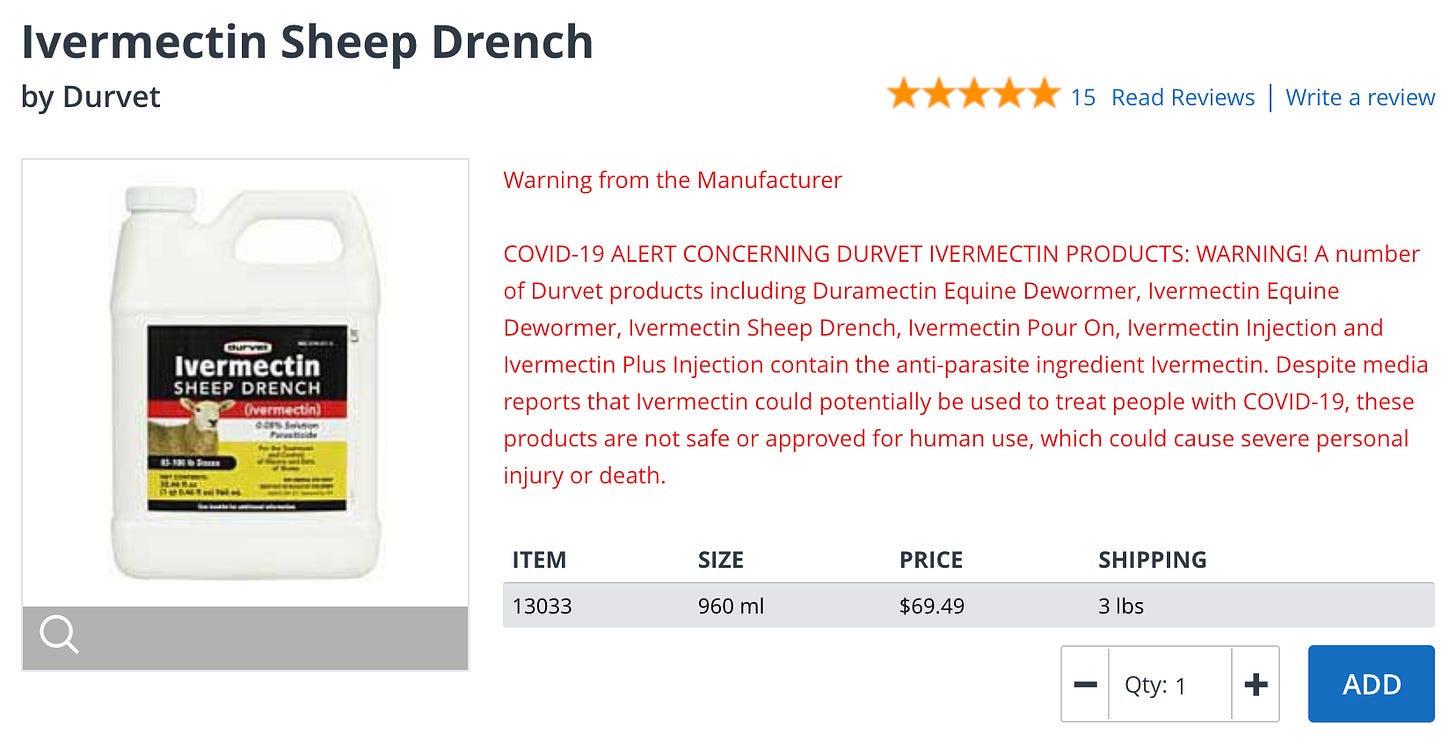
So how many people do you know that have rushed online or to their neighborhood farm and feed supply store to pick up a liter of Sheep Drench or perhaps 50 ml of injectable large livestock ivermectin?
You would think from the “alert” on the FDA twitter feed that many are seeking out the animal version of Ivermectin as an alternative to the various injectable compounds produced by Pfizer, Moderna, and J&J.
This is not true.
There may be some individuals that are frustrated with the regulation that they see with large livestock Ivermectin and there may also be some that are frustrated at the supply chain limitations being imposed on distribution of ivermectin that is designed for human consumption. The livestock versions of Ivermectin are also strangely restricted and regulated within California.
So you can’t even get these “animal products” in California based on these restrictions. So where are the offenders obtaining the animal products and administering them?
Here is the warning label for the “sheep drench”…
IVERMECTIN SHEEP DRENCH
Durvet
(ivermectin)
0.08% Solution Parasiticide
For the Treatment and Control of Worms and Bots of Sheep
Consult your veterinarian for assistance in diagnosis, treatment, and control of parasitism.
FOR ORAL USE IN SHEEP ONLY. NOT FOR HUMAN USE.
PRODUCT DESCRIPTION: Ivermectin Sheep Drench is a ready-to-use, free-flowing solution of ivermectin. It is formulated to deliver the recommended dose rate of 0.2 mg ivermectin per 1 kg body weight given orally at a volume of 3.0 mL per 26 lbs body weight.
INDICATIONS:
Ivermectin Sheep Drench provides treatment and control of adult and fourth-stage larvae of the following parasites: Gastrointestinal Roundworms - Haemonchus contortus, Ostertagia circumcincta, Trichostrongylus axei, T. colubriformis, Cooperia curticei, Nematodirus spathiger, N. battus, and Oesophagostomum columbianum; Lungworms - Dictyocaulus filaria; and all the larval stages of Nasal Bot - Oestrus ovis. It also provides treatment and control of adult forms only of the following Gastrointestinal Roundworms - Haemonchus placei, Cooperia oncophora, Strongyloides papillosus, Oesophagostomum venulosum, Trichuris ovis, and Chabertia ovina.
DOSAGE AND ADMINISTRATION: Ivermectin Sheep Drench may be used in any standard drenching equipment or in any equipment which provides a consistent dose volume.
Ivermectin Sheep Drench is administered orally at a dose of 3.0 mL (2.4 mg ivermectin) per 26 lbs body weight or 200 mcg ivermectin per kilogram of body weight.
Coughing may be observed in some animals during and for several minutes following drenching.
Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
RESIDUE WARNING: Do not treat sheep within 11 days of slaughter.
The Safety Data Sheet (SDS) contains more detailed occupational safety information.
To report adverse effects in users, to obtain an SDS, or for assistance call 1-800-650-4899.
PRECAUTIONS:
Ivermectin (ivermectin) Sheep Drench has been formulated for use in sheep only. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
OTHER WARNINGS: Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers.
Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance.
Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd/flock, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method).
A decrease in a drug’s effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
Keep this and all drugs out of reach of children.
Refrain from smoking and eating when handling. Avoid contact with eyes. Immediately wash hands and any spills on the skin with plenty of soap and water following use.
Restricted Drug (California) - Use Only as Directed.
Environmental Safety: Studies indicate that when ivermectin comes in contact with the soil, it readily and tightly binds to the soil and becomes inactive over time. Free ivermectin may adversely affect fish and certain water-born organisms on which they feed. Do not permit water runoff from feedlots to enter lakes, streams, or ground water. Do not contaminate water by direct application or by the improper disposal of drug containers. Spills should be contained and soaked up with absorbent towels or into loose soil. Gloves should be worn to prevent skin exposure. All the collected materials (contaminated towels and soil), as well as all empty drug containers, should be placed in an impervious film (plastic) bag and disposed of by incineration or in an approved landfill.
STORAGE INFORMATION:
Store at 68° - 77° F (20° - 25° C). Excursions between 59° - 86° F (15° - 30° C) are permitted.
For Animal Use Only
Keep Out of Reach of Children
Approved by FDA under ANADA # 200-327
Manufactured For:
DURVET, INC. Blue Springs, MO U.S.A.
www.durvet.com
Net Contents:
NDC
8 fl oz (240 mL)
20-100 lb Doses
30798-637-12
ISS21XB01
32.46 fl oz (1 qt 0.46 fl oz) 960 mL
83-100 lb Doses
30798-637-16
ISS21XB01